Decoding the NASH to MASH Transition
The liver disease landscape has recently undergone a significant transformation with the reclassification of Nonalcoholic Steatohepatitis (NASH) to Metabolic Dysfunction-Associated Steatohepatitis (MASH). This renaming more accurately reflects the metabolic origins of the disease, highlighting its connection to obesity, diabetes, and metabolic syndrome. While Non-Alcoholic Fatty Liver Disease (NAFLD) includes a broader range of conditions, MASH specifically focuses on the inflammatory and fibrotic progression of liver damage.
MASH Treatment Landscape: Patient Pool and Market Outlook
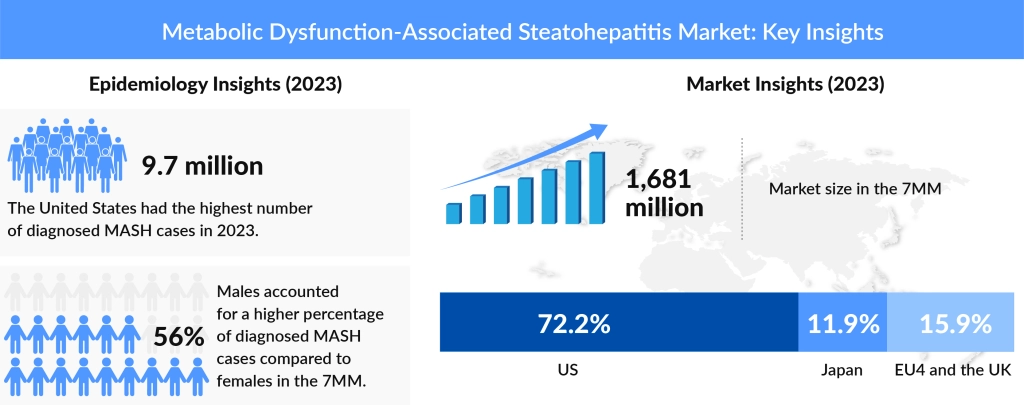
The prevalence of MASH is on the rise, affecting millions worldwide. Formerly recognized as NASH, this disease remains a major health burden due to its progressive nature, which can ultimately lead to cirrhosis and hepatocellular carcinoma. As awareness and diagnosis of NASH grew, the demand for treatments has also been rising. With the transition to MASH, pharmaceutical companies are realigning their research strategies. Analysts forecast an increased demand for MASH treatments, with FDA approvals playing a pivotal role in market growth.
Current Drugs in the Pipeline for NASH and MASH
The NASH pipeline has seen a surge of investigational therapies, many of which are now being repurposed for MASH treatment. Key upcoming therapies include drugs targeting metabolic pathways, anti-fibrotic agents, and anti-inflammatory mechanisms. Numerous companies are pursuing innovative treatments, with several late-stage candidates awaiting FDA approval in the near future.
Transitioning to MASH: A Paradigm Shift in Research and Development
Pharmaceutical companies are adapting their NASH treatment strategies to fit the new MASH classification. Clinical trials are increasingly focused on addressing metabolic dysfunction, allowing for more targeted and effective therapeutic approaches. This shift is expected to enhance drug efficacy, improve patient outcomes, and facilitate regulatory approvals.
Challenges and Opportunities in MASH Treatment
Despite promising progress, challenges such as disease heterogeneity, the absence of validated biomarkers, and regulatory hurdles persist. However, the transition from NASH to MASH presents exciting opportunities for the development of novel drugs and precision medicine approaches.
Conclusion
The shift from NASH to MASH marks a pivotal turning point in liver disease research and treatment. As the NASH market transitions, FDA-approved drugs and ongoing clinical trials for MASH treatment are shaping the future of care, offering hope for better patient outcomes and more effective therapies.
Latest Reports Offered By Delveinsight
ANCA Vasculitis Market | Familial Lipoprotein Lipase Deficiency Market | Focal Segmental Glomerulosclerosis Market | Herpes Labialis Market | Langerhans Cell Histiocytosis Market | Opioid-Related Disorders Market | Pancreatic Endocrine Tumor Market | Pelvic Organ Prolapse Market | Polymyalgia Rheumatica Market | Recurrent Herpes Labialis Market | Secondary Progressive Multiple Sclerosis Market | Spinocerebellar Ataxia Market | Surgical Bleeding Market | Tonic Clonic Seizure Market | Urology Ultrasounds Devices Market | Uveitis Market | Carbapenem-Resistant Enterobacteriaceae Infection Market | Glioblastoma Multiforme Market | Pediatric Brain Tumor Market | Rubella Market | Smoking Cessation and Nicotine Addiction Market | Wilms Tumor Market
About Delveinsight:
DelveInsight is a leading provider of market research and consulting services, specializing in the life sciences and healthcare industries. Our insights support pharmaceutical, biotechnology, and medical device companies in navigating competitive environments and achieving long-term success.
Contact Information: Kanishk
Email: kkumar@delveinsight.com